Step-by-step tutorial
Step 1
Wash your penny in soapy water to get it clean. Dry it off with a paper towel.

Step 2
Carefully cut one aluminum foil rectangle (1/2 inch x 8 inches or 1.3 x 20.3 cm) and fold it lengthwise three times until you get a sturdy 1/2 inch by 8-inch strip.

Step 3
Place your lemon on its side on a plate and (with a grownup's help) cut two 3/4 inch (1.9 cm) slits in the middle of the lemon, about 1/2 inch (1.3 cm) apart. Make sure the cuts are deep enough to expose the juice.If the skin of the lemon is very thick, ask a grownup to cut away some of the skin.

Step 4
Push one penny into the first slit until you are sure that the penny is touching the lemon juice.

Step 5
Slide the aluminum strip into the second cut until you are sure that the strip is touching the lemon juice. Trim down the strip to fit in the second cut if it's too long.
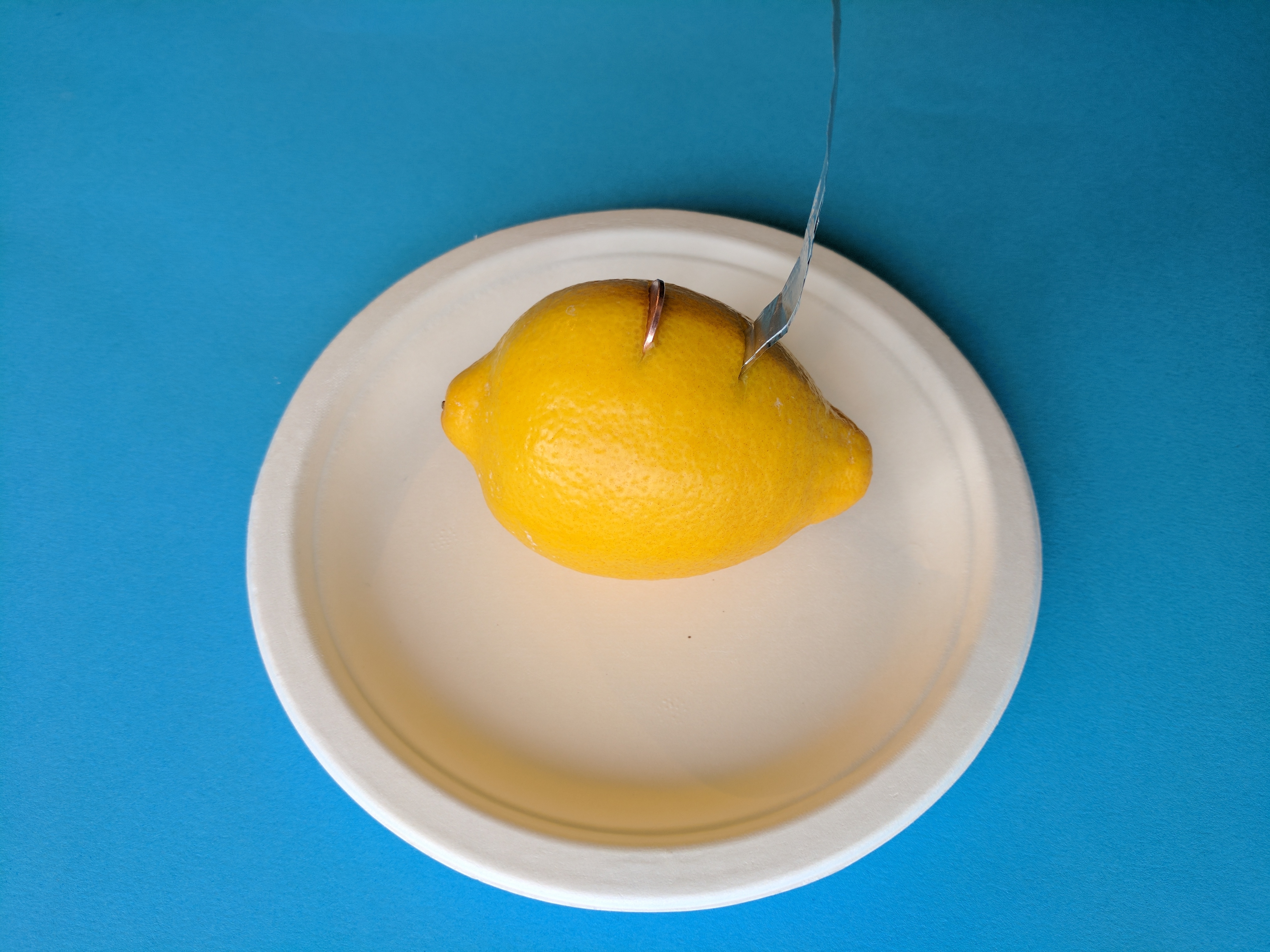
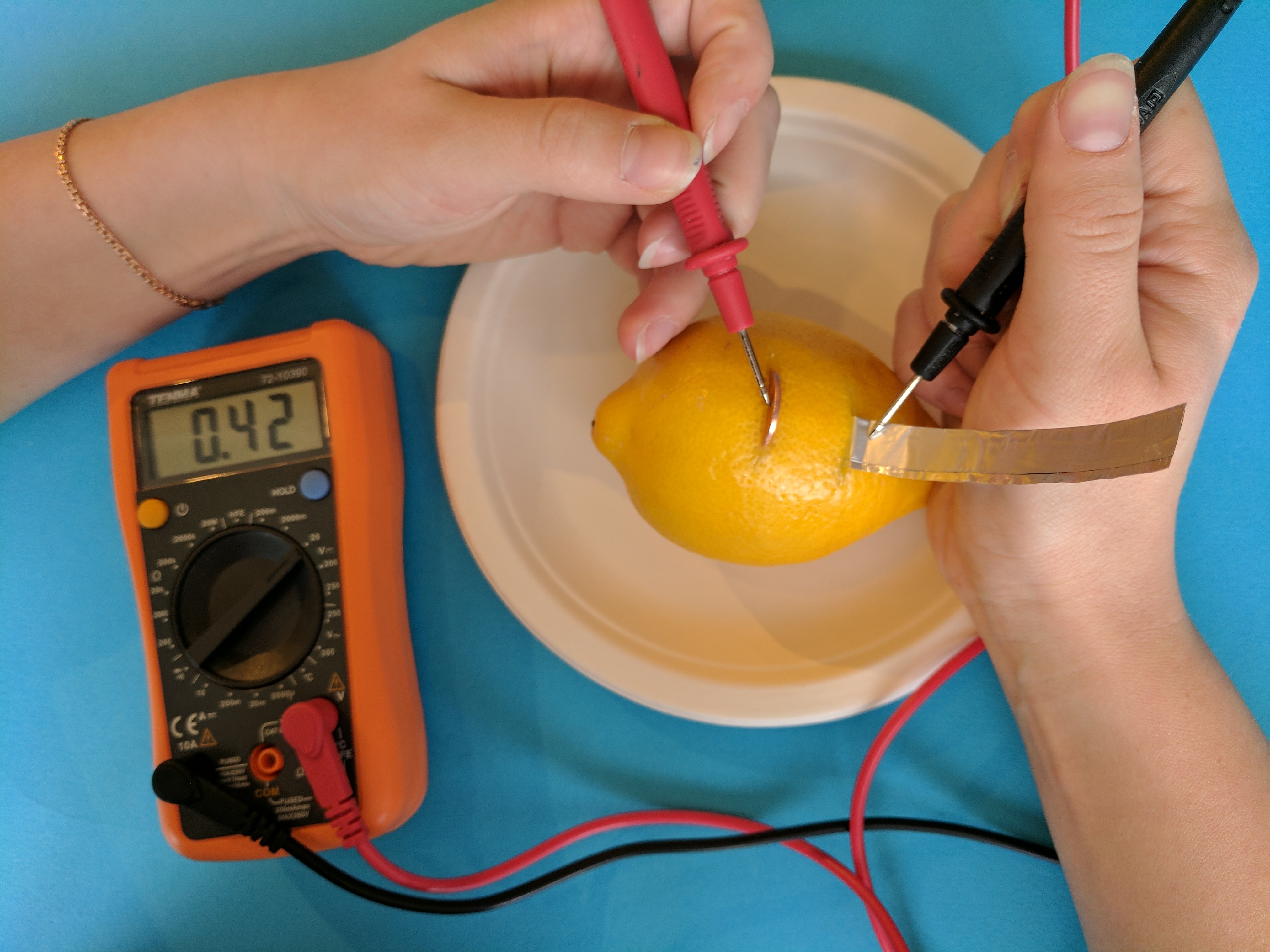
Step 6
You've made a working battery! In order to measure the voltage produced, touch one node of the multimeter to the penny and the other to the aluminum strip. How many volts are you able to produce?
Learn more
What's going on?
The lemon battery is a good example of an electrochemical cell, which converts chemical energy into electricity. This electricity results from the flow of electrons from the aluminum foil (the negative terminal) towards the copper penny (the positive terminal). Electrons prefer to flow in this direction because copper attracts electrons more strongly than aluminum. However, these electrons can’t escape the aluminum on their own (this is why you don’t get electricity when you simply touch aluminum foil to a penny). Here’s where the lemon comes into play. Citric acid inside the juice of the lemon reacts with the aluminum foil, freeing up electrons in the foil. Now these electrons can easily flow across a wire towards the copper, creating the electric current that shows up on the multimeter.
