Step-by-step tutorial
Step 1
Carefully put your egg into a jar and fill the jar about three-quarters full with the egg completely submerged in the vinegar.

Step 2
You should be able to see bubbles form around the egg immediately. Where do you think they come from? Wait 48 to 72 hours. We found it exciting to check in on the egg as we went as the egg grows and changes over time.

Step 3
After waiting, use a spoon to take out the egg. Carefully rinse it thoroughly under the faucet using warm water.

Step 4
Your egg is now ready to bounce! Hold your egg about 3 inches from the table and gently let go. If bounced too hard the egg will break. This means you'll get to see the membrane of the egg!

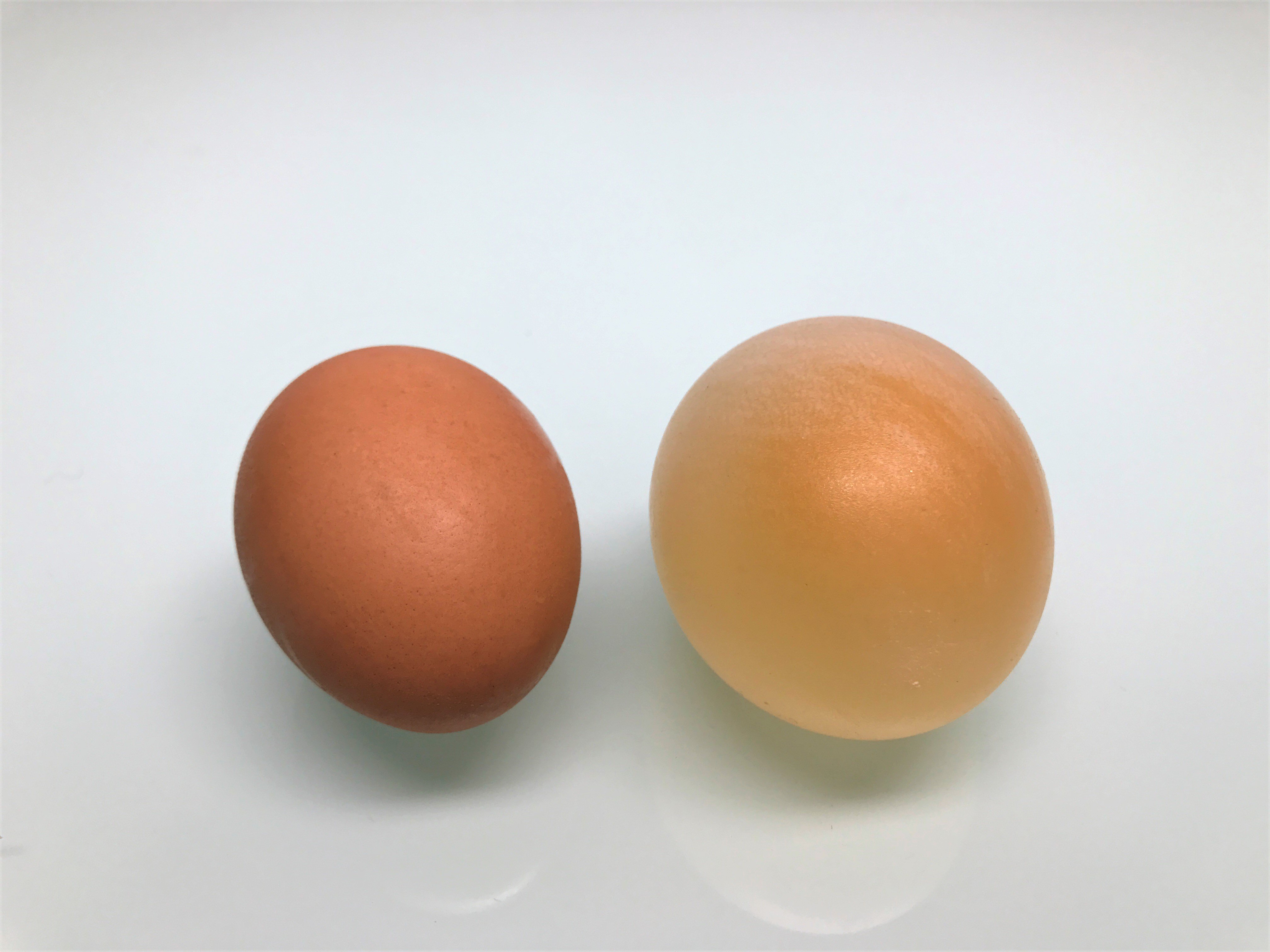
Final result!
Compare your transformed egg to a regular egg. What do you think happened to the eggshell? What other differences can you observe? What's Going On? If you look closely at the egg while it's submerged in the vinegar, you can see bubbles forming on the surface. Those bubbles are full of carbon dioxide, just like the bubbles in a glass of soda. You're seeing a reaction between a compound in the eggshell (calcium carbonate) and an acid in the vinegar (acetic acid). This reaction creates carbon dioxide (and some other things) and breaks down the eggshell in the process. The membrane underneath the shell doesn't react, so it's left behind. Once the shell is completely gone, all that's left is the flexible membrane, giving you a bouncy "rubber" egg!